1 Physician, Specialist in Cardiology – Centro Cardiovascular Nueve de Julio. 2 Physician, Specialist in Cardiovascular Surgery – Sanatorio Junín. 3 Physician, Specialist in Interventional Cardiology – Centro Cardiovascular Nueve de Julio.
Corresponding author’s address
Dr. Cardinali Ré Braian A.
Phone: 2317431038
Postal address: Hipólito Irigoyen 1068 – CP 6500- 9 de Julio- Provincia de Buenos Aires.
E-mail
Received on July 15, 2022 Accepted after review on August 10, 2022 www.revistafac.org.ar
There are no conflicts of interest to disclose.
Keywords:
Myocardial infarction.
Pseudoaneurysm.
Echocardiography
ABSTRACT
Left ventricular pseudoaneurysm is an uncommon clinical entity, mainly caused by acute
myocardial infarction (AMI). This is a severe complication and potentially deadly. However, in
exceptional cases, the rupture is contained by organized hematoma, pericardium and fibrous
tissue, avoiding the fatal outcome and allowing surgical or endovascular management in selected
cases. Here is the case of a large pseudoaneurysm diagnosed in a patient with recent infarction..
INTRODUCTION
Cardiac pseudoaneurysm is a rare entity, but with great clinical relevance due to its high probability of rupture and death. This entity is defined as an incomplete rupture of the heart’s wall, where the pericardium remains sealing the rupture, and a new chamber develops, communicated with the ventricular chamber through a narrow orifice[1].
In contrast with true aneurysms, which always contain myocardium in their wall, pseudoaneurysm’s wall is constituted by pericardium and organized hematoma, lacking the elements of original ventricular wall, myocardium and endocardium[1].
IThe most frequent etiology is usually a complication of acute myocardial infarction (AMI) (55%); but it may also present as a complication of heart surgery (33%), subsequent to ventriculotomy, or after mitral valve replacement at the level of posterior annulus, and after aortic valve replacement at subaortic level. Acute pseudoaneurysm after AMI is an extremely unstable entity, and with very high mortality[2].
The consequence of this type of lesions is fatal for patients, since pseudoaneurysm rupture is usually followed by sudden hypovolemic shock, leading quickly to electromechanical dissociation and death of patients.
The average of presentation is around 60 years. They occur more frequently in the male gender. The most frequent symptoms at the time of presentation are heart failure and chest pain; sudden cardiac death occurs in 3%; but up to 12% were a finding in asymptomatic patients. The presence of heart murmur was found in more than two thirds of cases.
With regard to basic supplementary methods, most patients have abnormal electrocardiograms and chest X-rays. Persistent ST-segment elevation is observed in 20%, along with unspecific alterations in the ST-T segment[3].
In this report, the clinical case of a male 71-year-old patient is presented. The patient presented subacute ventricular pseudoaneurysm as a complication of non-revascularized anterior AMI. It was solved through endovascular access.
CLINICAL CASE
Male, 71-year-old patient, with multiple cardiovascular risk factors (hypertension, dyslipidemia and severe smoker), with history of recent myocardial revascularization surgery, who went to the ER brought by a local ambulance service due to syncope.
The patient reported history of oppressive precordial pain, of 8/10 intensity, radiating to the inferior maxillary bone, of 45 minutes of duration, having occurred 15 days before the cardiological appointment, which did not lead to a consultation due to the epidemiological situation of COVID-19.
At the time of physical examination, the patient was in a regular general state, asymptomatic for angina, with functional class III of dyspnea, of the New York Heart Association (NYHA), severely hypotensive, with signs and symptoms of heart failure, and symptoms compatible with cardiogenic shock.
At the level of the cardiovascular system, normal heart sounds, and no extra sounds. Apical S3. Peripheral filiform pulse. Jugular vein distension 2/3, no inspiratory collapse. Presence of bibasilar crackles.
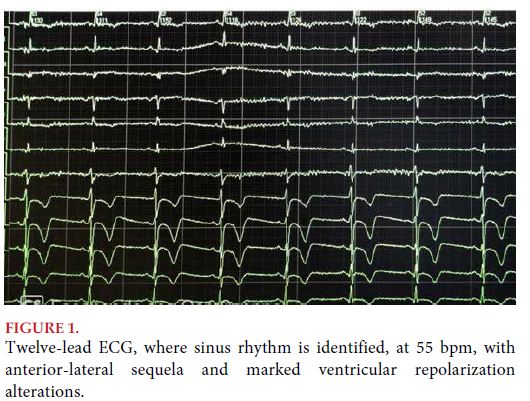
Twelve-lead electrocardiogram was conducted, which showed sinus rhythm at 70 bpm, extensive anterior sequela with deep negative T waves (Figure 1).

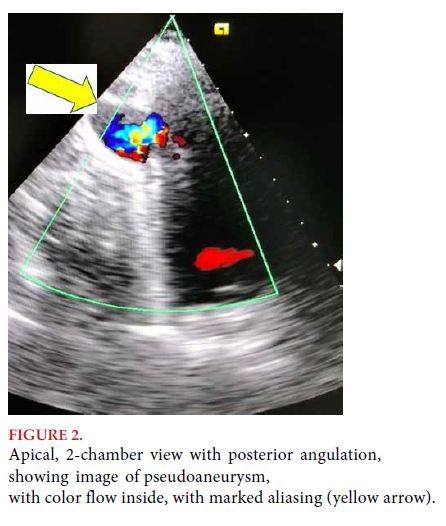
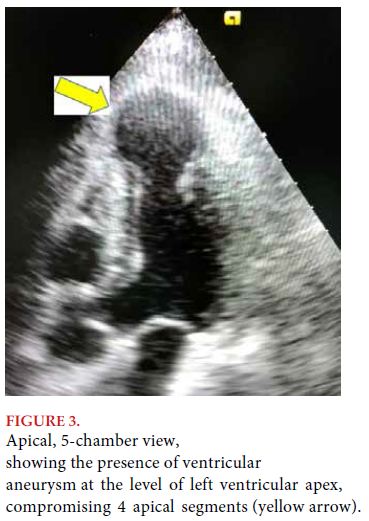
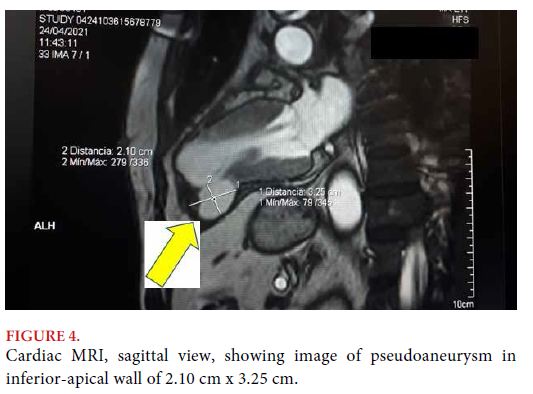
DISCUSSION
Cardiac pseudoaneurysm is a rare entity, but with great clinical significance due to a high probability of rupture and death. This entity is defined by incomplete rupture of all cardiac wall layers, where the pericardium remains sealing the rupture, and a new chamber develops, communicated with the ventricular chamber by a narrow orifice[1].
There is a classification for the development of pseudoaneurysm according to the time when diagnosis is made: acute (diagnosed up to two weeks after infarction), subacute (between two weeks and three months) and chronic (after three months subsequent to infarction)[4].
Pseudoaneurysm in the left ventricle is usually the result of left ventricular wall rupture, after acute myocardial infarction (AMI) or heart surgery, with or without valve replacement. The risk of rupture is around 30 to 45% in the first year, and surgical option is associated to 20 to 35% mortality[1,2,3,4,5]. Percutaneous therapy seems a feasible, effective and possibly the safest option[6,7].
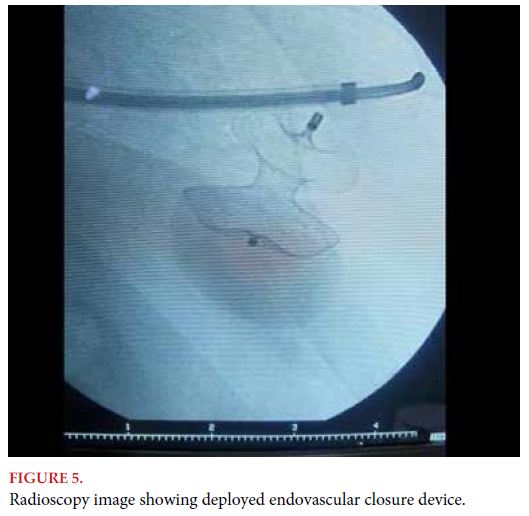
Clinically, left ventricular pseudoaneurysm may present in an asymptomatic patient, with chest pain, or it may even cause symptoms of congestive heart failure. Left ventricular pseudoaneurysm was historically treated surgically. However, it usually appears in previously operated patients, with surgical adhesions that make intraoperative management difficult, and with comorbidities that lead to a high procedure-related morbidity, as in the case presented here. From this scenario, percutaneous treatment by femoral or transapical access is gaining ground, and has become a feasible alternative with satisfactory results[6,7].
CONCLUSIONS
In the post-thrombolytic era, there has been a significant reduction in mechanical complications as reported in numerous series and investigations in this field, but with the appearance of the COVID-19 pandemic, patients go less often to emergency services or outpatient clinics, so a discrete increase has been observed on these complications secondary to the absence of a timely therapy in these patients.
Left ventricular pseudoaneurysm is a rare but deadly mechanical complication in post-AMI patients, who are usually patients in high surgical risk, so currently and once again, endovascular resolution is being chosen for this type of complications.
BIBLIOGRAPHY