Review Article
Atrial fibrillation and valvular regurgitation: another example of continuum
and self-perpetuation
Juan I. Cotella1,2, Carla Pinna2,
Aldo Prado2.
1University of Chicago, Chicago, USA. 2 Centro Privado de Cardiología, Tucumán, Argentina.
Corresponding author’s address
Dr. Juan I. Cotella.
Postal address: 400 East Randolph, 60601, Chicago, IL, USA.
E-mail
INFORMATION
Received on September 30, 2021
Accepted after review
on July 9, 2022 www.revistafac.org.ar
There are no conflicts of interest to
disclose.
Keywords:
Atrial fibrillation.
Valvular regurgitation.
Echocardiography
ABSTRACT
Atrial fibrillation is the most frequent arrhythmia among adults, and it is associated to a substantial
risk increase of cerebrovascular events and heart failure. Among the multiple conditions related
to this arrhythmia, functional mitral and/or tricuspid regurgitation, secondary to atrial fibrillation
and atrial dilation, have received much less attention. Although this entity may require a different
approach than the rest of the valvular diseases, international guidelines are still not clear about it.
The goal of this review is to provide a brief description of the complex pathophysiological mechanisms
involved, highlighting the role of echocardiography in its diagnosis and comprehension, and
emphasizing on the presence of therapeutic conundrums still unanswered.
Atrial fibrillation (AF) is the most common arrhythmia in adult patients[1]. In turn, the increase in population’s age at world level is a fact that allows us to estimate that the prevalence of AF will double in the next 50 years, affecting by then, more than 5.6 million people just in the United States[2,3].
Likewise, mitral regurgitation (MR) in a moderate or major degree constitutes the most frequent valve disease in the United States, affecting 2 million people in 2002, waiting to double this figure around year 2030[4,5].
In patients with persistent long-standing AF or persistent AF, the increase in left atrium size may lead to mitral annulus dilatation and posterior MI[5]. This series of events leads to an entity known as atrial functional mitral regurgitation (AFMR)[6].
Many publications have elegantly described the complex mechanisms involved in this condition, as well as the essential role of echocardiography in its diagnosis and evaluation[5,6,7,8].
Just as with AFMR, the presence of functional tricuspid regurgitation (TR) has also been described in patients with AF[9]. Just as with mitral level, the presence of TR secondary to tricuspid annular dilatation would generate right atrial enlargement and perpetuation of tricuspid remodeling, even when the right ventricular (RV) function and morphology remain preserved[9]. However, the presence of moderate and severe TR has been related to an increase in the risk of mortality, regardless of pulmonary pressure and right ventricular function values[10].
Where does the problem start? Although AF is the axis in this issue, the usefulness of LA size as predictor of adverse results in valve disease has already been described[11]. Its clear role as a risk factor for the appearance and installation of AF, heart failure and even death by cardiovascular cause and by all causes has been proven[12]. As stated previously, the impact on atrioventricular valves is the consequence of atrial dilatation in patients with AF, causing both the mitral and the tricuspid annuli to increase in size, to acquire a rounder and flatter shape, and to lose contractility[9,13].
As already presented in the title, and running the risk of oversimplifying, interpreting this condition as a continuum is considered useful from a practical point of view, and a self-perpetuation between arrhythmic phenomena and structural alterations (both atrial and valvular) (Figure 1).
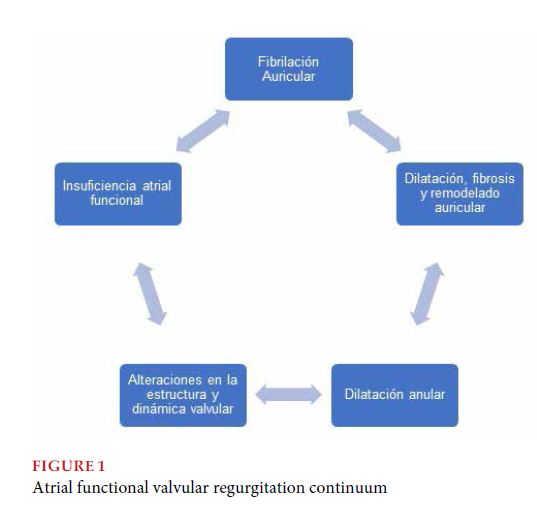
Does AF duration matter? Deferm et al, have reported a prevalence of 6-7% of AFMR in patients with lone AF[14]. However, other studies point out that in patients with lone or paroxysmal AF and with no primary valve pathology associated, the presence of MR has generally been mild or even absent in spite of the presence of annular dilatation, so it has been suggested that annular dilatation in these patients would not be enough to explain per se, the degree of severity of regurgitation[12,13,15].
How are valves analyzed? Is it just a problem of the annulus? In patients with AFMR, the analysis of quantification of atrial ventricular morphology and function, of valvular anatomy and geometry, as well as the degree of severity and mechanism of regurgitation by 2-dimensional echocardiography (2DE) and three-dimensional echocardiography (3DE) are fundamental[16,17].
When faced with this complex structure, the dynamic and relationships of the mitral valve apparatus (underscoring the particular saddle-shaped annulus), 2DE, mental reconstructions and geometric and spatial assumptions of observers using this technique constitute a potential source of error in measurements and estimations[16,17]. The evaluation of the mitral valve apparatus by 3DE allows to define with more accuracy the extension, location and mechanism of mitral valve pathology and facilitates the interpretation and subsequent communication of the findings by surgeons/interventional physicians at the time of making therapeutic decisions[16].
Joint efforts with the industry have enabled the development of software capable of providing 3D models obtained from 3DE. Thus, it is possible to obtain detailed information on static parameters (annulus dimensions and shape, aorto-mitral angle measurement, valve length measurement), and dynamic changes in the mitral valve apparatus along the cardiac cycle (Figure 1). The multiplicity of mechanisms entailed in the wide range of mitral valve disease, and particularly in AFMR, highlight the role of techniques as the one described above, which allow for a better and greater understanding of the pathology, so that we can make better decisions.
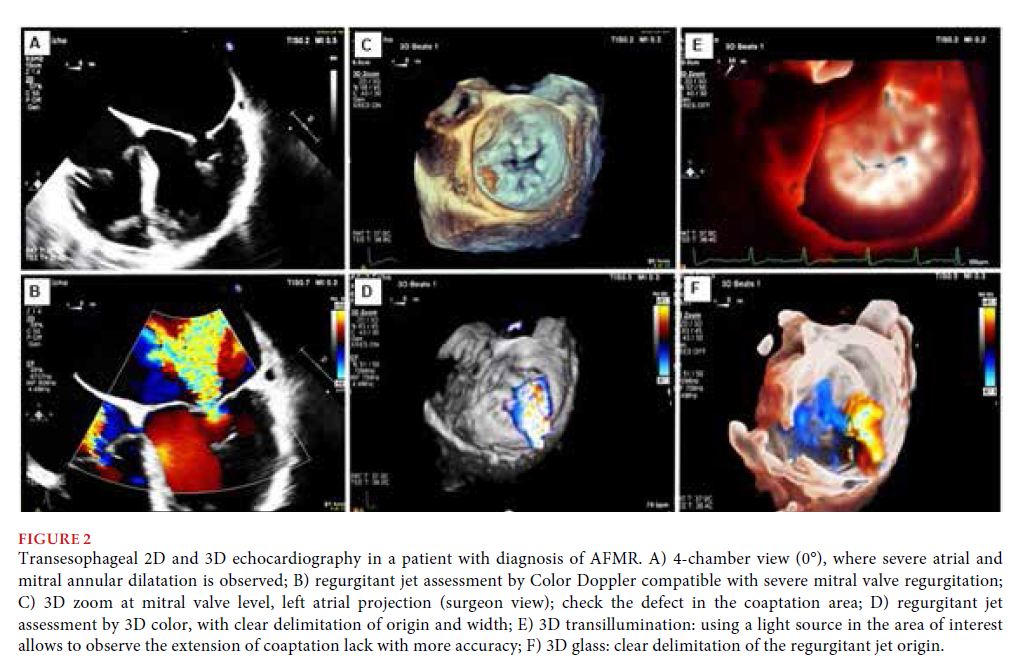
Specifically, the analysis and interpretation of atrioventricular valves functional regurgitation is challenging. We should take into account that in these patients, regurgitant jet generally adopts a tangential direction on the coaptation area, with an arrangement that may lead to underestimating quantitative parameters of severity[18]. Transesophageal echocardiography, mainly if transducers are available with the capacity of performing 3DE, allows to solve this problem, having shown advantages to perform more accurate measurements of the degree of severity and mechanism of MR, in comparison to other transthoracic techniques[7,8,19].
As already stated, 3DE and its modalities (transillumination, glass, etc.) provide different tools to prevent the geometric assumptions of 2DE[20]. Some examples are the visualization of valves from a surgical perspective (surgeon’s view) (Figure 2), 3D measurement and quantification of the vena contracta, the regurgitant jet and the effective regurgitant orifice area, with more accuracy than conventional 2DE techniques[20]. 3DE has allowed us to observe that the vena contracta does not present a perfectly circular shape, fundamentally in patients with functional regurgitation, and that such assumption leads to mistakes in measurements, potentially affecting decision making[16]. It is important to clarify that advancements in echocardiography should not be interpreted as replacing one method by another, but as a permanent optimization of the technique proper, where knowledge on the advantages and limitations of each modality, as well as the criteria to use it, will determine the best results.
Likewise, although evidence has shown that LA dimensions and function improve after ablation, atrial volumes estimated by 3DE were less than those reported by angiography or electroanatomic mapping[6,21,22]. These discrepancies between methods also suggest that using 3DE may improve the measurements of atrial morphology[23].
However, techniques like tissue Doppler or speckle-tracking have proven to be sensitive to detect early or subclinical modifications in LA function[11]. In comparison to patients with paroxysmal AF, those with persistent long-standing AF have shown lower values of LA strain, suggesting that besides annular dilatation, a certain degree of atrial myopathy would be involved in the development of AFMR[24,25].
And how are these patients treated? Gertz et al. have shown that cardioversion and sinus rhythm restitution may reduce atrial size and MR severity in more than 70% of cases of AFMR[26]. Another similar publication has described a decrease in TR severity and reverse remodeling of right chambers, after radiofrequency ablation in patients with persistent AF[27]. However, patients that presented AF recurrence after ablation did not present considerable changes in annular dimensions in spite of a reduction in atrial size, and 80% of these patients still presented significant MR[26]. This may indicate that sinus rhythm restoration, although necessary, may not be enough for all patients with AFMR, so interventions at valve level could be required.
Silbiger et al. have proposed multiple mechanisms involved in AFMR, including the presence of valve tethering, repositioning of posterior valve against the left ventricular posterior wall, annular-papillary imbalance, decrease in mitral valve contractility and insufficient valve growth to prevent regurgitation (endothelial-mesenchymal transformation)[18].
Although limited by availability, percutaneous valve repair techniques (Transcatheter edge-to-edge repair [TEER]) are attractive in certain groups, particularly an elderly and/or fragile population. However, severe atrial dilatation present in patients with AFMR is not the most favorable scenario for the implementation of these techniques. Thus, in the case of being necessary and when surgical risk is not excessive, along with AF ablation, these patients could obtain better results by direct surgical interventions (e.g., annuloplasty) on the mitral valve[28].
And what happens at tricuspid level? How can it be handled? Just as with the mitral valve, the presence of atrial functional tricuspid regurgitation (AFTR) has been described, even with a prevalence close to 25% in patients with AF of more than 10 years of duration[9]. Studies using 2DE and 3DE have described annular dilatation as a determining factor for coaptation deficit and morphological changes in the tricuspid valve, entailing the appearance and development of AFTR[29,30].
One of the hypotheses proposed is that, when presenting a supporting structure less rigid than the mitral annulus, the tricuspid annulus presents more predisposition to dilatation secondary to atrial enlargement. It has been described that to generate a significant degree of TR, a tricuspid annulus dilatation of just 40% is necessary; while at mitral level, a dilatation of more than 70% is needed to justify the appearance of significant MR[29].
Patients with AFTR may present a rapid progression of TR severity, with a subsequent right chamber dilatation[28]. In a multivariate analysis, Guta et al. have shown that right atrial volume is independently associated to the tricuspid annulus area, both being predictor elements for TR worsening in patients with AF[31].
A diastolic diameter of the tricuspid annulus of more than 40 mm (21 mm/m2) (measured from a 4-chamber apical view) is an indication for surgical intervention (annuloplasty) in patients who will undergo left chamber surgery, even when TR severity is mild at the time of intervention[28]. Thus, the assessment of the tricuspid valve is crucial, as it is a scenario where 3DE allows to perform a proper and thorough analysis of the complex tricuspid valve apparatus[20]. The capacity of this technique to observe and understand the relationships between the tricuspid annulus and the right chambers have highlighted the determining role of right atrial enlargement in annular dilatation, regardless of heart rhythm and hemodynamic conditions[32].
Finally, although ventricular function is usually preserved in these patients, a prevalence of AFMR of more than 50% has been reported in patients with heart failure and preserved ejection fraction[14]. We should take into account that a complete and optimized treatment of heart failure should be the primary goal, before deciding on any type of surgical intervention in patients with functional valvular heart disease. In turn, it has been observed (retrospective study, 71 patients) that the only predictive factor for TR severity regression was improvement in left ventricular systolic function[33].
In this brief review, we have attempted to emphasize the role of AF (particularly long-standing AF) in the installation and perpetuation of functional mitral and tricuspid regurgitation. However, the questions asked will probably remain without a definitive answer. It is still not clear what conditions proper of patients entail a greater risk of developing this condition; an even therapeutic approach is not clear either. With an underestimated prevalence and so many interrogations with no answer, there is a single certainty: the number of patients with AF will continue increasing, and thus associated valve disease, and its constant self-perpetuation. Consequently, solidifying clinical suspicion and its identification fully comprehending its complexity, the undeniable benefit of treating the arrhythmic substrate, and the proven usefulness of echocardiography will become the foundations to identify and guide the treatment of this condition.
BIBLIOGRAPHY
- Benjamin EJ, Muntner P, Alonso A, et al. American Heart Association
Council on Epidemiology and Prevention Statistics Committee and
Stroke Statistics Subcommittee (2019). Heart Disease and Stroke Statistics-
2019 Update: A Report from the American Heart Association. Circulation
2019; 139: e56 – e528.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation
in adults: national implications for rhythm management and
stroke prevention: the Anticoagulation and Risk Factors in Atrial Fibrillation
(ATRIA) Study. JAMA 2001; 285: 2370 – 2375..
- Enriquez-Sarano M, Akins C, Vahanian A. Mitral regurgitation. Lancet
2009; 373: 1382 – 1394.
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases:
a population-based study. Lancet 2006; 368: 1005 – 1011.
- Ring L, Dutka, DP, Wells FC, et al. Mechanisms of atrial mitral regurgitation:
insights using 3D transesophageal echo. Eur Heart J Cardiovasc
Imaging 2001; 15: 500 – 508.
- Muraru D, Andrada-Camelia G, Badano L, et al. Functional regurgitation
of atrioventricular valves and atrial fibrillation: an elusive pathophysiological
link deserving further attention. JASE 2019; 33: 42 - 53.
- Ito, K, Abe Y, Takahashi, Y, et al. Mechanism of atrial functional mitral
regurgitation in patients with atrial fibrillation: A study using threedimensional
transesophageal echocardiography. J Cardiol 2017; 70:
584 – 590.
- Mahino-Otsuka T, Yoshihiro S, Ishizu T, et al. Novel Mechanistic Insights
into Atrial Functional Mitral Regurgitation: A 3-Dimensional
Echocardiographic Study. J-Stage 2016; 80: 2240 – 2248.
- Muraru D, Guta AC, Badano LP, et al. Functional Regurgitation of
Atrioventricular Valves and Atrial Fibrillation: An Elusive Pathophysiological
Link Deserving Further Attention. J Am So Echocardiogr
2020; 33: 42 – 53.
- Wang N, Fulcher J, Abeysuriya N, et al. Tricuspid regurgitation is associated
with increased mortality independent of pulmonary pressures
and right heart failure: a systematic review and meta-analysis. Eur
Heart J 2019; 40: 476 – 484.
- Rosca M, Lancellotti P, Popescu BA, et al. Left atrial function: pathophysiology,
echocardiographic assessment, and clinical applications. Heart
2011; 97: 1982 – 1989.
- Abhayaratna WP, Seward JB, Appleton CP, et al. Left atrial size: physiologic
determinants and clinical applications. J Am Coll Cardiol 2006;
47: 2357 – 2363.
- Kihara T, Gillinov AM, Takasaki K, et al. Mitral regurgitation associated
with mitral annular dilation in patients with lone atrial fibrillation: an
echocardiographic study. Echocardiography 2009; 26: 885 – 889.
- Deferm S, Bertrand PB, Vandervoort PM, et al. Atrial Functional Mitral
Regurgitation: JACC Review Topic of the Week. J Am Coll Cardiol 2019;
73: 2465 – 2476.
- Otsuji Y, Kumanohoso T, Yoshifuku S, et al. Isolated annular dilation
does not usually cause important functional mitral regurgitation: comparison
between patients with lone atrial fibrillation and those with
idiopathic or ischemic cardiomyopathy. J Am Coll Cardiol 2002; 39:
1651 – 1656.
- Lang RM, Badano LP, Mor-Avi, V, et al. Recommendations for cardiac
chamber quantification by echocardiography in adults: an update from
the American Society of Echocardiography and the European Association
of Cardiovascular Imaging. J Am Soc Echocardiogr 2015: 28: 1 –39.
- Zoghbi WA, Bonow RO, Lang R, et al. Recommendations for Noninvasive
Evaluation of Native Valvular Regurgitation: A Report from the
American Society of Echocardiography Developed in Collaboration
with the Society for Cardiovascular Magnetic Resonance. J Am Soc
Echocardiogr 2017; 30: 303 – 371.
- Silbiger JJ. Mechanistic insights into atrial functional mitral regurgitation:
Far more complicated than just left atrial remodeling. Echocardiography
2019; 36: 164 – 169.
- Huang DQ, Cui CY, Zhang J, et al. Effects of nonvalvular atrial fibrillation
on the structure and function of mitral valves (a STROBE-compliant
article). Medicine 2018; 97: e11643.
- Addetia K, Lang RM, Badano LP. 3-Dimensional Echocardiographic
Analysis of the Tricuspid Annulus Provides New Insights into Tricuspid
Valve Geometry and Dynamics. JACC Cardiovasc Imaging 2019;
12: 401 – 412.
- Miyasaka Y, Tsujimoto S, Maeba H, et al. Left atrial volume by real-time
three-dimensional echocardiography: validation by 64-slice multidetector
computed tomography. J Am Soc Echocardiogr 2011; 24: 680 – 686.
- Marsan NA, Tops LF, Holman ER. Comparison of left atrial volumes
and function by real-time three-dimensional echocardiography in patients
having catheter ablation for atrial fibrillation with persistence of
sinus rhythm versus recurrent atrial fibrillation three months later. Am
J Cardiol 2008; 102: 847 – 853.
- Lang RM, Badano LP, Tsang W, et al. European Association of Echocardiography
EAE/ASE recommendations for image acquisition and display
using three-dimensional echocardiography. J Am Soc Echocardiogr
2012; 25: 3 – 46.
- Kuppahally SS, Akoum N, Burgon NS, et al. Left atrial strain and strain
rate in patients with paroxysmal and persistent atrial fibrillation: relationship
to left atrial structural remodeling detected by delayed-enhancement
MRI. Circ Cardiovasc Imaging 2010; 3: 231 – 239.
- Tamargo M, Obokata M, Reddy Y, et al. Functional mitral regurgitation
and left atrial myopathy in heart failure with preserved ejection fraction.
Eur J Heart Fail 2020; 22: 489 – 498.
- Gertz ZM, Raina A, Saghy L, et al. Evidence of atrial functional mitral
regurgitation due to atrial fibrillation: reversal with arrhythmia control.
J Am Coll Cardiol 2011; 58: 1474 –1481.
- Itakura K, Hidaka T, Nakano Y. Successful catheter ablation of persistent
atrial fibrillation is associated with improvement in functional tricuspid
regurgitation and right heart reverse remodeling. Heart Vessels
2020; 35: 842 – 851.
- Otto CM, Nishimura RA, Bonow RO. 2020 ACC/AHA Guidelines for
the Management of Patients with Valvular Heart Disease: Executive
Summary: A Report of the American College of Cardiology/American
Heart Association Joint Committee on Clinical Practice Guidelines. Circulation
2020; 143: e35 – e71.
- Spinner EM, Lerakis S, Higginson J, et al. Correlates of tricuspid regurgitation
as determined by 3D echocardiography: pulmonary arterial
pressure, ventricle geometry, annular dilatation, and papillary muscle
displacement. Circ Cardiovasc Imaging 2012; 5: 43 – 50.
- Utsunomiya H, Itabashi Y, Mihara H, et al. Functional Tricuspid Regurgitation
Caused by Chronic Atrial Fibrillation: A Real-Time 3-Dimensional
Transesophageal Echocardiography Study. Circ Cardiovasc Imaging
2017; 10: e004897.
- Guta A, Badano LP, Muraru D et al. The pathophysiological link between
right atrial remodeling and functional tricuspid regurgitation in
patients with atrial fibrillation: A three dimensional Echocardiography
Study. J Am Soc Echocardiogr 2021; 34: 585 – 594.
- Muraru D, Addetia K, Lang R, et al. Right atrial volume is a major determinant
of tricuspid annulus area in functional tricuspid regurgitation:
a three-dimensional echocardiographic study. Eur Heart J - Cardiovasc
Imaging 2021; 22: 660 – 669.
- Cho JP, Kim HK, Park JC, et al. Predictors of reversible severe functional
tricuspid regurgitation. J Cardiol 2016; 68: 419 – 425.


