1 Centro Integral de Arritmias, Tucumán, Argentina. 2 Clínica Incor, Santa Cruz, Bolivia. 3 Medicine School student, Universidad San Pablo de Tucumán, San Miguel de Tucumán, Tucumán, Argentina.
Corresponding author’s address
Dr. Luis Aguinaga Arrascue
Postal address: Santiago del Estero 157, 5to piso. 4000, San Miguel de Tucumán, Tucumán, Argentina.
E-mail
Received on August 23, 2022 Accepted after review on August 24, 2022 www.revistafac.org.ar
There are no conflicts of interest to disclose.
Keywords:
Cardiac Pacing.
Leadless Pacing.
Argentina
ABSTRACT
Cardiac stimulation is a widely growing specialty during the last decades. The number of implanted cardiac devices is higher; but it is not rare to find patients presenting contraindications and difficulties for the implantation of a device. The cardiac leadless devices technology is a promising option that offers better safety with less implant-related complications. Besides, it is capable of maintaining atrioventricular synchrony with a single-chamber device.
In this brief communication, the authors report the first 4 implants of MICRA AV devices in Argentina.
INTRODUCTION
Cardiac pacing advances at a startlingly rapid progress, with an increasing number of implantations of devices of cardiac pacing, due to a great variety of indications. National and international clinical practice guidelines, in their last versions, have as a novelty the inclusion of “leadless” pacemaking. The Consenso Argentino de Cardiología sobre Marcapasos y Resincronizadores (Argentine Consensus of Cardiology on Pacemakers and Resynchronizers) focuses on the results from the MICRA STUDY, not making recommendations for the implant of these devices. We should remark that this document was published in 2020 and does not include the MICRA AV device with atrioventricular synchrony[1].
The 2021 ESC Guidelines on Cardiac Pacing and Cardiac Resynchronization acknowledges the capacity of generating AV synchrony with the VDD algorithms of the new leadless pacemakers in up to 90%; besides the low rate of complications (2.7%) during the implantation of these devices by experienced hands, which are lower than those reported in the implantations of conventional pacing devices (6 to 8%). Although the complications may appear less frequently in experienced hands, it is acknowledged that there are severe complications (perforation, tamponade, vascular complications, ventricular arrhythmias and death). This document does make recommendations for the implantation of these devices[2].
It is advised to consider leadless pacemakers as an alternative for transvenous pacemakers when there is no venous access in the upper limbs, or when the risk of pacing of the device is high, as in patients with previous infection and patients in hemodialysis. Recommendation class: IIa, level of evidence B[2].
Pacemakers could be an alternative to standard single-lead ventricular pacing, taking into account life expectancy and a strategy of shared decision-making. Recommendation class: IIb, level of evidence C[2].
REQUIREMENT FOR LEADLESS PACING
In spite of the great advancements in cardiac pacing technology over recent decades, conventional pacemakers maintain the same basic structure as its origins back in the 1970s (extravascular pulse generator connected to the myocardium by transvenous electrodes). Electrodes are the weakest spot in this circuit, being in a hostile environment, having to withstand mechanical tension from its vascular environment and shoulder movement. Besides, the whole circuit constitutes a mechanism that predisposes to infections[3].
It is for all this, that the requirement to develop a self-contained pacemaker has been pursued since the 1970s (previously limited by batteries and their duration), being a reality nowadays with the MICRA system having been approved by the FDA in 2016[3]..
LEADLESS DEVICE IMPLANTATION
An ideal leadless pacemaker is compact, light, small enough to allow multiple implants in the future, easily implantable and removable. The device should not be related to the genesis of thromboembolic events or ventricular ectopy. The device’s delivery system should not damage the valvular heart apparatus; and finally, the incidence of cardiac perforation should be equal or less than that of conventional devices[3]..
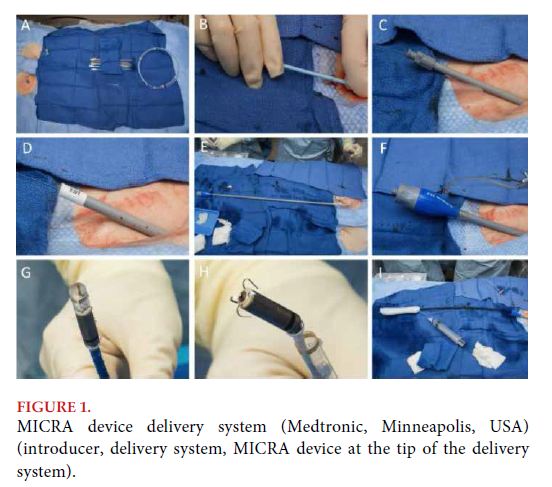
Leadless devices validated currently meet all the previously mentioned requirements. Implantation is made by femoral access and the devices have myocardial attachment systems. The MICRA device (Medtronic, Minneapolis, USA) uses a 27 French femoral introducer requiring heparin during implantation, and has an attachment system based on self-expandable pins that ensure fixation (Figure 1)[4].

ATRIOVENTRICULAR SYNCHRONY
The single-chamber nature of leadless technology limited these devices to pacing in VVI or VVIR, lacking AV synchrony. Thanks to the technological innovation of using accelerometers in the device, it is possible to achieve the identification of atrial kick and achieving high percentages of synchrony, thus increasing the range for leadless technology implants[5].
The MARVEL 2 (Micra Atrial tRacking using a Ventricular accELerometer 2) study evaluated the performance of technology and algorithms for AV synchrony of the MICRA AV device. There were 75 patients included in 12 centers, from which 40 were in sinus rhythm with complete AV block, increasing AV synchrony from 26% in VVI to 89.2% (mean 94%) in VDD; with no reports of pauses or tachycardic events by oversensing[5].
FIRST MICRA AV IMPLANTATIONS IN ARGENTINA
On August 16 and 17, 2022, at the Centro Integral de Arritmias Tucumán (CIAT), the first 4 implantations of the MICRA AV device were conducted in Argentina. Next, we present the cases and corresponding images.
CASE 1
Male, 63-year-old patient with history of hypertension, diabetes mellitus, chronic kidney disease, in hemodialysis, with symptomatic sick sinus syndrome. Implantation performed in 40 minutes, with no complications. Threshold after delivery system withdrawal of 0.25 V per 0.4 ms, impedance of 678 ohms. Twenty-four hours after the implantation, programming was implemented in VDD pacing mode, obtaining atrioventricular synchrony with similar threshold and impedance parameters.
CASE 2
Male, 77-year-old patient with history of sick sinus syndrome, paroxysmal atrial fibrillation with low ventricular response. The implantation was made with no complications in 45 minutes. A pacing threshold of 0.38 V per 0.4 ms and impedance of 712 ohms were obtained. He remained in atrial fibrillation in 24-hour telemetry, with successful ventricular capture and threshold and impedance parameters similar to those of the implantation (Figure 2).
CASE 3
Male, 61-year-old patient with history of hypertension, previous subclavian vein cannulation, with chronic venous obstruction, and symptomatic sick sinus syndrome. The implantation was made in 43 minutes, with no complications. The threshold after withdrawal of the delivery system was 0.25 V per 0.4 ms. Impedance of 767 ohms. Twenty-four hours later, programming was implemented of VDD pacing mode, obtaining atrioventricular synchrony with similar threshold and impedance parameters (Figure 3).

CASE 4
Female, 73-year-old patient, with history of symptomatic sick sinus syndrome, with implant of conventional dual-chamber pacemaker, complicated with pacemaker pocket infection 1 month after implantation, percutaneous withdrawal of electrode 1 month and a half after implantation. The implantation was made in 50 minutes with no complications. Threshold after delivery system withdrawal of 0.38 V per 0.4 ms. Impedance of 856 ohms. Twenty-four hours after the implantation, programming was implemented in VDD pacing mode, obtaining atrioventricular synchrony with similar threshold and impedance parameters.
CONCLUSIONS
Cardiac pacing has advanced encouragingly with the advent of leadless pacing, obtaining more safety and less complications in the implant when in experienced hands. The future is promising about the long-term performance of these devices, besides continuous technological advancements that predict leadless devices implantation for cardiac resynchronization and more battery duration.
BIBLIOGRAPHY